Next-generation mobile monitoring
Cardiac clinicians and patients have faced significant challenges with post-surgery remote patient monitoring. Clunky hardware, inconsistent and unsuccessful data flow, and confusing or inefficient user experience issues have led to delayed decision-making by clinicians and frustrated patients who are already under mental and physical stress due to cardiac conditions.
01
Human-centered research and insights informed a rapid prototype that quickly validated our approach
02
Successful Class II medical device FDA submission and market rollout
03
Authored numerous documents included in the FDA and IEC 62304 regulatory submission processes
Implementation of this product required a skill set and integration of technology new to our team. Nerdery has been key in helping us develop and integrate these areas.
— Medical Device Client
A solution to an outdated healthcare device
Nerdery collaborated with the global MedTech company to build a next-generation mobile monitoring device from scratch. We began with a high-level concept of the scripting engine designs and produced a rapid prototype to validate the approach. Partnering with the client’s human factors, systems and marketing teams, we were able to identify a need for digital solutions that would:

- Provide patients with a simple, secure, easy-to-use remote monitoring experience that makes them feel safe, supported and empowered.
- Enable the implant support team to quickly and reliably set up and activate the ICM device and patient monitoring.
- Provide physicians with improved detection algorithms and more successful and regular patient data transmissions.
- Provide physicians with streamlined patient registration and tracking process.
- Enable a clinic to run a monitoring service line effectively with an efficient, scalable infrastructure in a fast-growing segment.
- Provide hardware diagnostics for insights into how the implantable cardiac monitor device performs in the field.
Taking a human-centered approach, we developed two mobile applications — one for patients and one for clinicians.
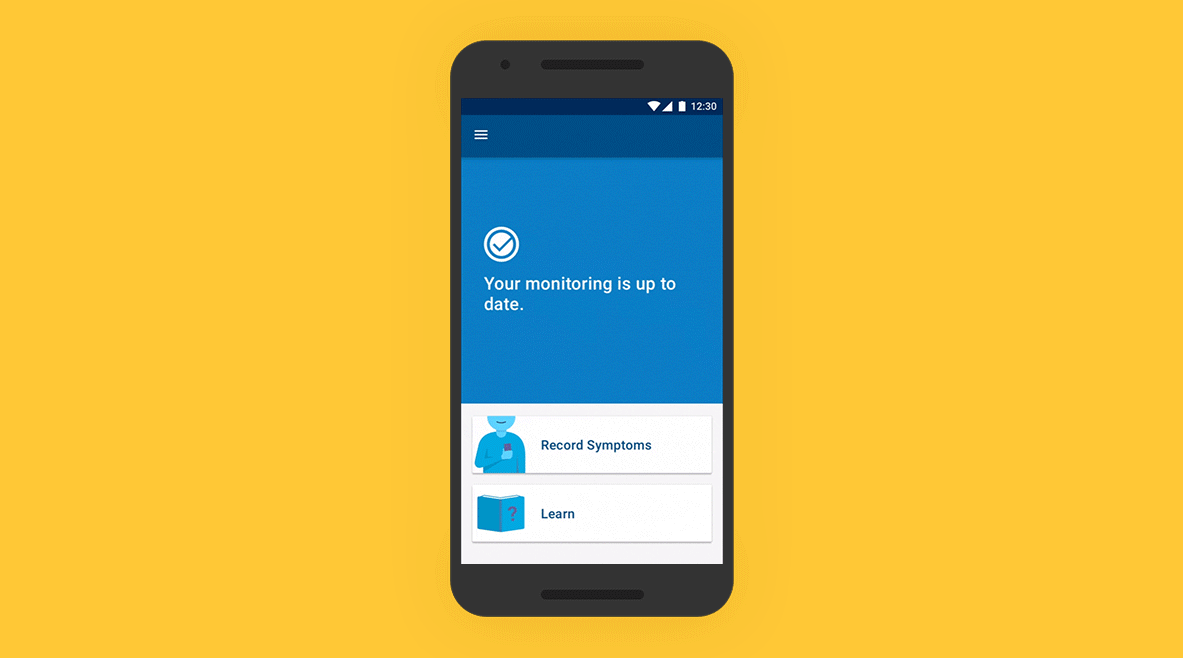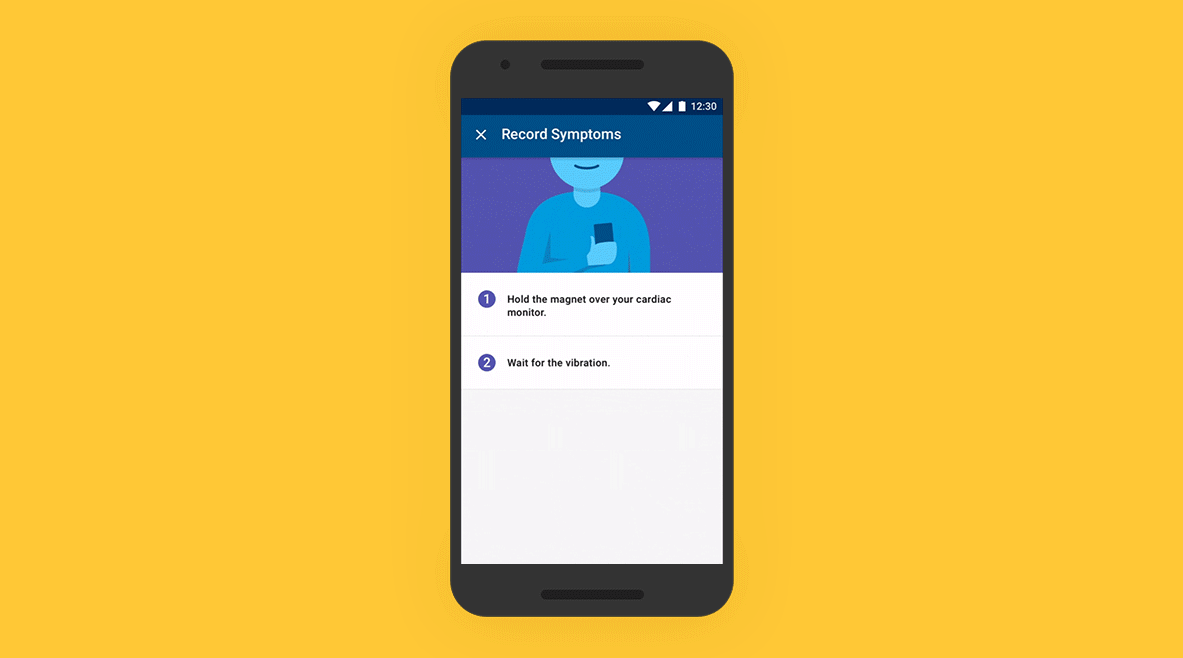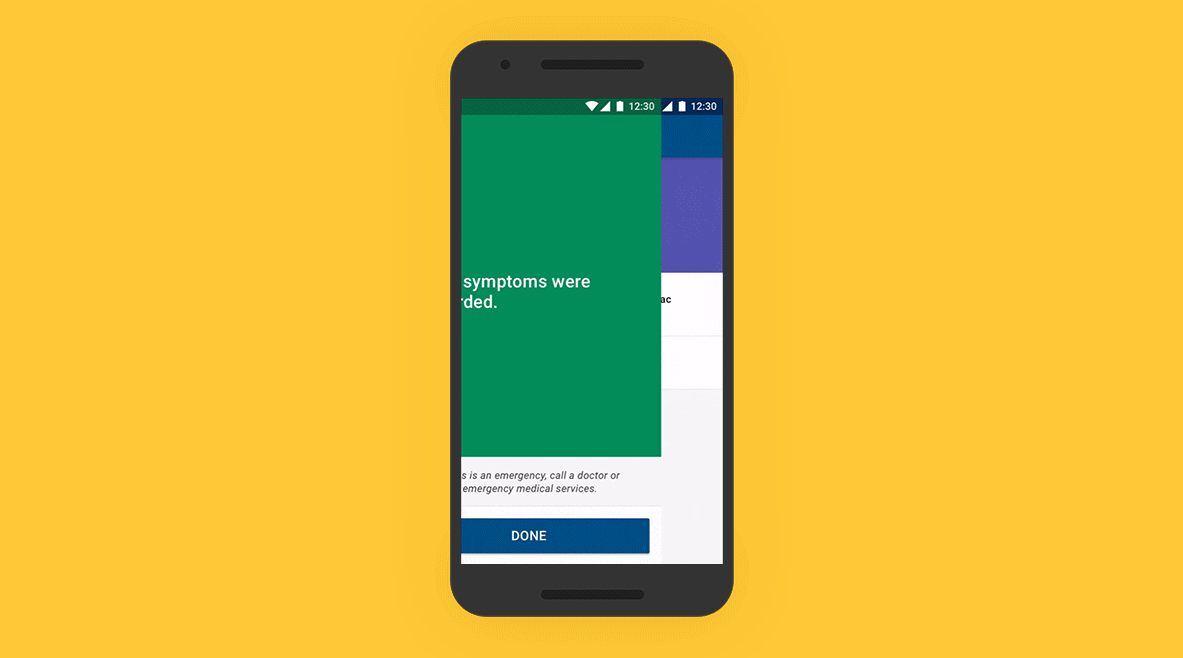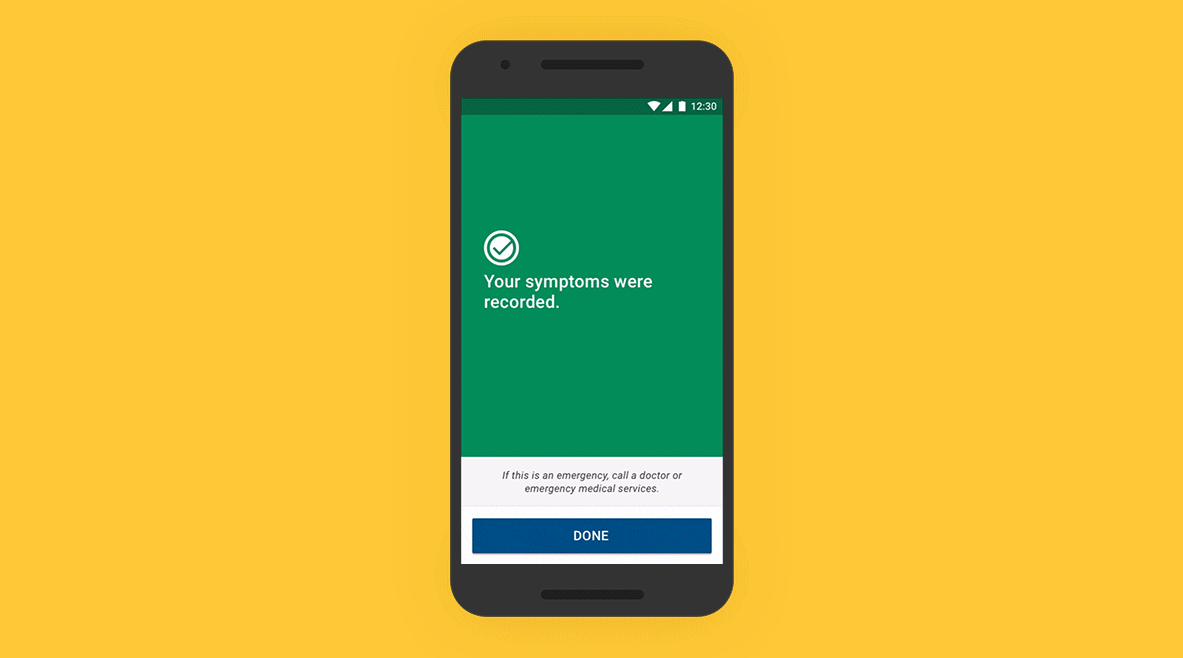
01 - Patient Application
On an easy-to-use app, patients track their symptoms, which are then mapped to the Bluetooth-transmitted data from their ICM. A simple setup process and intuitive daily symptom monitoring experience build confidence in patients, even if they are unfamiliar with using a smartphone.
02 - Clinician Application
With the clinician-facing app, clinicians are empowered with streamlined patient registration and tracking process, as well as more successful and regular patient data transmissions, including a dual-stage algorithm that sends an alert when it detects and then verifies potential arrhythmia in a patient.
The multi-year effort led to a successful Class II medical device FDA submission and market rollout.